On Monday, April 22nd, a seminar titled “Breast Cancer Precision Prevention – Presenting Results from Sweden, Estonia and Portugal” was held at Uppsala University Hospital. The seminar showcased preliminary results and explored opportunities for the implementation of breast cancer precision prevention in healthcare.
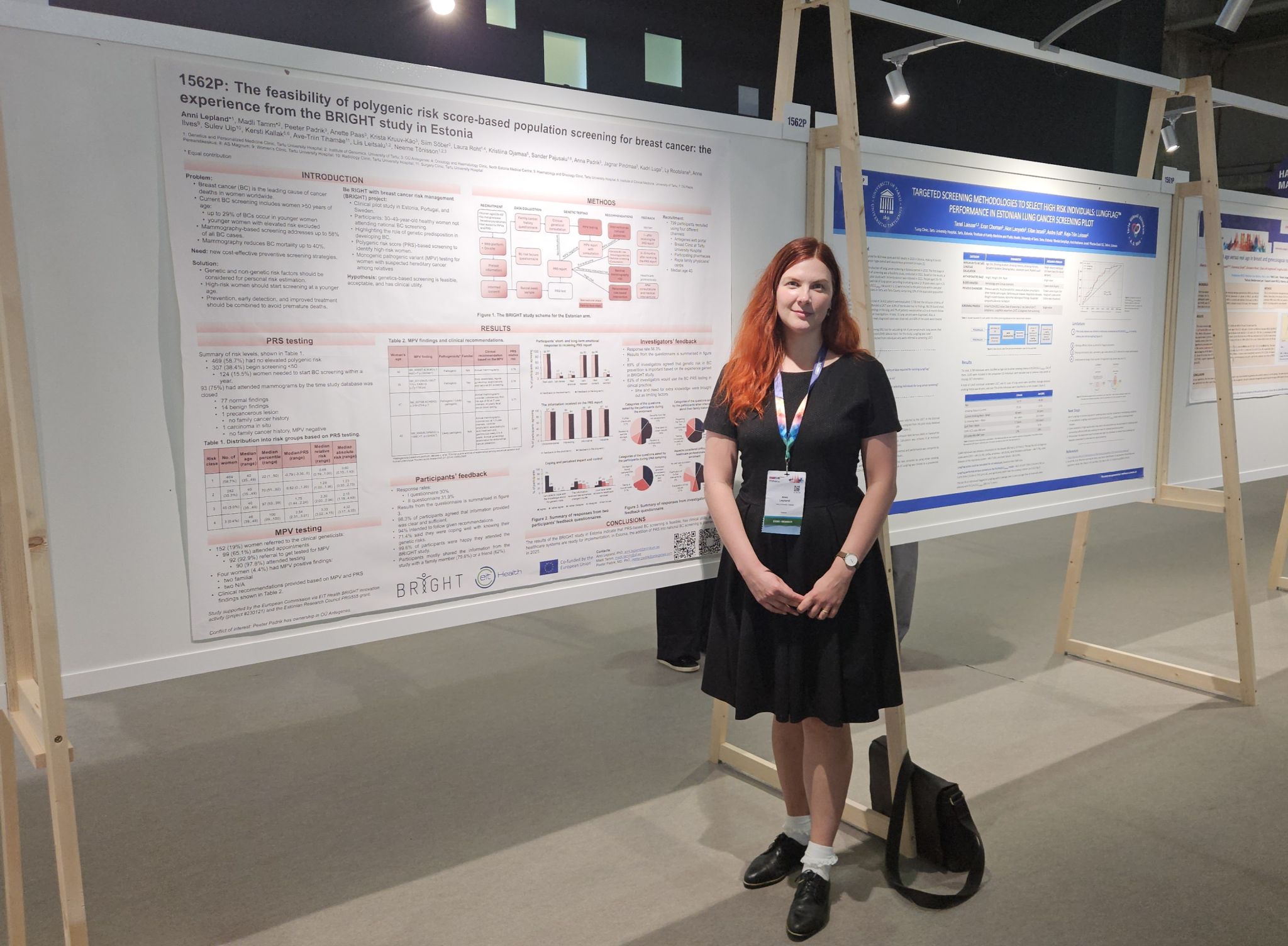
EIT Health-funded BRIGHT project investigates stratified breast cancer screening based on genetic risk estimated with polygenic risk scores (PRS). The purpose of the study is to improve opportunities for early detection of breast cancer based on genetic risk, as opposed to today’s standardised mammography screening based solely on age. Across all countries participating in the BRIGHT project, women between the ages 30-49 years are assessed for their genetic predisposition to breast cancer and personalised risk-based screening recommendations are provided. The project uses innovative clinical-grade polygenic risk score test AnteBC, which is developed by a health technology company – Antegenes.
The BRIGHT study (Be RIGHT with Breast Cancer Risk Management) was initiated in 2022 in Sweden, Estonia and Portugal. In Sweden and Estonia, the clinical implementation study is finished and 1600 women were recruited in total. Volunteer recruitment in Portugal ends in May 2024. The logistical and ethical considerations and information flow are also assessed in the clinical pilot. In addition, cost-effectiveness of precision breast cancer prevention is evaluated.
Preliminary results from Sweden
In Sweden, recruitment utilized media channels, websites and an email address for participant communication. Recruitment was conducted through a form in the digital registration system, which included information about the study, survey data and contact information. By the end of March, the planned number of study participants had been reached, as approximately 800 individuals expressed interest in participating. Each participant received an AnteBC test kit, including instructions and a return envelope, which they could easily do as a home based test.
Upon analysis, participants were categorised into three groups based on the PRS test results: those with low to average risk (less than or up to the population average risk RR = <1.5), slightly elevated risk (RR = 1.5 – 2.7) and elevated risk (RR = ≥ 2.7). It revealed that 16% of participants had a slightly elevated risk, while 83% exhibited a lower or average risk and 1% had an elevated risk. Prof. Inna Feldman, health economist and project lead for the study in Region Uppsala, explained that women identified with a low to average risk were provided with a report and a letter containing recommendations. Those with slightly elevated or elevated risk levels received a report, a recommendation letter, and an offer for a consultation.
At the seminar, Dr. Filipa Sampaio, health economist at Region Uppsala, gave a presentation titled “Cost-effectiveness analysis of a polygenic risk-tailored breast cancer screening – the BRIGHT project experience”, highlighting preliminary results for the Swedish setting. The cost-effectiveness analysis examined the potential costs and benefits of various scenarios including PRS-based screening, focusing on personalised services for women in different risk categories. Alongside more frequent mammography screening for women at elevated risk of breast cancer, a scenario where women at low risk would commence screening at a later stage than currently recommended was also assessed.
Inna Feldman, project leader from Region Uppsala, highlighted the results from the follow-up survey. Feedback was collected from all participants, resulting in a response rate of 60%. “92% of respondents indicated that they were glad that they had the opportunity to participate in the BRIGHT study, and 87% reported feeling calm regarding the results,” highlighted Prof. Inna Feldman. Additionally, she noted that 75% of respondents found the explanations provided about the genetic risk and the clinical recommendations to be understandable.
Strong interest from women in Portugal and Estonia
The Portuguese branch of the BRIGHT study has recruited 653 volunteers so far; during the study period one case of invasive breast cancer was diagnosed. There has been a lot of interest in study participation and genetic testing. The study has surprisingly revealed that many women have already had mammograms before screening. However, regular self-examination was not common among participants, especially among those from high-risk families.
The study highlighted a need for increased awareness and understanding of genetic testing, as well as clear communication of results by healthcare professionals. “Moving forward, our focus is on integrating polygenic risk scores into clinical practice and disseminating study findings to relevant stakeholders,” said Prof. Luis Costa who is the project lead for the study in Portugal, emphasising the potential implications for national screening programs and cancer management protocols.
The Estonian branch of the clinical trial is concluded, and Estonian results will soon be published. Recruitment for the study was conducted through four different channels: Antegenes’ online portal offering home-based testing, selected pharmacies, Breast Clinic of Tartu University Hospital, and family physician services. The preliminary findings indicate that out of the 800 participants, 15.9% had a recommendation to initiate their mammography screening already this year. “This means that the polygenic risk score testing had an immediate effect on their follow-up,” said the BRIGHT project activity leader Prof. Neeme Tõnisson from the University of Tartu. Within the Estonian arm of the project, 127 women underwent mammograms, with 16 benign changes identified and 8 women requiring further investigations.
Patient organisations advocating for breast cancer precision screening
At the seminar, there was also a discussion involving participation from patient organisations, as well as the Uppsala Breast Cancer Association and Region Uppsala. The participants in the discussion highlighted the importance of population-wide precision breast cancer screening services, the significance of educating women about PRS to improve health literacy, and emphasised the need to present the results to politicians and decision-makers.
Kim Höglund from the Uppsala Breast Cancer Association emphasised the importance of young women having access to PRS testing, to facilitate early diagnosis. “Early diagnosis is crucial. It’s very important that everyone knows about this opportunity and extensive information campaigns should be conducted for that purpose.” Additionally, they underscored the importance of developing explanations of PRS test results tailored to different levels of health literacy. Patient representatives expressed strong support for the broad implementation and equal access to PRS testing. The seminar was also attended by representatives and members from the Karolinska Institute, the Swedish Regional Cancer Center, EIT Health, Uppsala University, the University of Tartu and the Estonian Cancer Center, in addition to the consortium members.
A more comprehensive analysis of the study results will be published in collaboration with the study’s international partners in peer-reviewed scientific journals. The consortium convened in Sweden to discuss immediate steps necessary for implementing precision prevention initiatives in project countries and to outline plans for ongoing research collaboration. On October 10-11th, a BRIGHT open seminar will take place in Portugal, where the initial results of the pilot study and preliminary cost-effectiveness analysis results in the Portuguese setting will be presented at North Lisbon University Hospital.
BRIGHT project partners are Region Uppsala, North Lisbon University Hospital Centre, University of Tartu, Tartu University Hospital, Antegenes, Estonian Health Insurance Fund, IESE Business School and GE HealthCare.