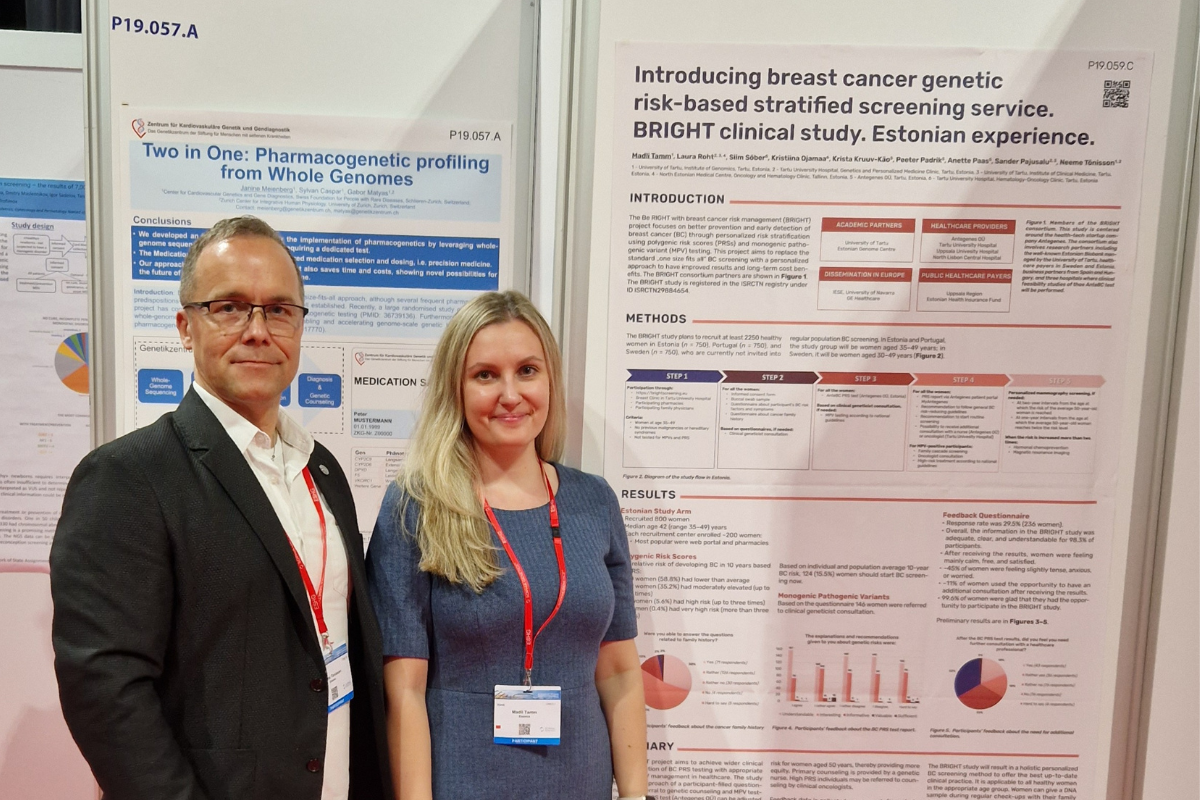
The breast cancer genetic risk-based stratified screening service was introduced at the European Society of Human Genetics Conference: preliminary results of the EIT Health BRIGHT project in Estonia.
The purpose of the international BRIGHT project is to implement genetics-based breast cancer precision prevention in European healthcare moving beyond the current standard screening which is offered based on age only. Preliminary results revealed that genetics-based breast cancer precision prevention is feasible using multiple channels for the inclusion of women, including telemedicine solution with home-based genetic testing.
The project uses innovative polygenic risk score test AnteBC, which is developed by health technology company Antegenes, combined with traditional monogenic pathogenic variant testing, and implements different channels for population-based genetic screening.
This innovative approach is led by the BRIGHT consortium, which includes the University of Tartu, Antegenes, Tartu University Hospital, IESE Business School, GE Healthcare, Uppsala Region, Uppsala University Hospital, North Lisbon Central Hospital and Estonian Health Insurance Fund.
The Estonian arm of the project targeted women at age 35-49, which is the age before current standard screening, but among whom 20% of breast cancer cases concur. 800 women with a median age of 42 (range 35-49) were included to the project using 4 different channels: Antegenes’ online portal using home-based testing, pharmacies, a breast clinic, and a family physician service. Approximately 200 women were registered in each channel.
The women who participated in the study took the genetic test AnteBC to assess the polygenic predisposition to breast cancer. AnteBC PRS test can be adjusted to a specific population’s BC risk data and the standard mammography screening starting age. BC screening for younger women is initiated at a time when the predicted individual risk equals the average risk for women aged 50 years, thereby providing more equity.
Results of the polygenic risk score testing with AnteBC test revealed that 124 (15.5%) women had breast cancer risk higher than average risk at age 50. These women should start breast cancer screening already now. Monogenic pathogenic variant (BRCA1, BRCA2, etc) testing were specified using questionnaires according to official indications for these tests.
236 women (29.5%) responded to the feedback questionnaire, which revealed that 99.6% of respondents were glad that they had the opportunity to participate in the BRIGHT project and that the information contained for breast cancer precision screening was adequate, clear and understandable to 98.3% of participants. 11% of the women who participated in the project took the opportunity to receive an additional oral consultation after receiving the results, others felt that written detailed recommendations were sufficient. Feedback data is collected anonymously from study participants and healthcare professionals to assess the feasibility, acceptability, and healthcare system readiness of a stratified cancer screening approach.
The integration of breast cancer polygenic risk score testing and parallel monogenic variants management in healthcare aims to establish a stratified cancer screening approach that ensures better patient outcomes, improved equity, and increased screening participation rates. A similar project is still being conducted in Portugal and Sweden.
The BRIGHT project will result in a holistic personalized BC screening method to offer the best up-to-date clinical practice. It is applicable to all healthy women in the appropriate age group. Women can give a DNA sample during regular check-ups with their family doctor or even comfortably at home. All of this should increase the screening participation rates and reduce BC-caused deaths. The solution is already implemented in Estonian healthcare as an opportunistic breast cancer precision screening.
BRIGHT project has been supported by the European Commission via EIT Health BRIGHT innovation activity (project #220720). For more information about the BRIGHT clinical study and breast cancer risk-based stratified screening service, please visit https://brightscreening.eu.